OPOD
What's New
Rays & Shadows
Water Droplets
Rainbows
Ice Halos
High Atmosphere
Tropopause
Ozone Layer
Mesosphere
Gravity Waves
Nacreous Clouds
Noctilucent Clouds
Rocket Trails
Airglow
Aurora
Zodiacal Light
Links & Resources
Search - Index
123456789012345678
| Ozone - The Stratosphere |
|
 |
| Total lunar eclipse March 3, '07 imaged in Germany by Eva Seidenfaden (atmospheric optics site) . The blue at the edge of the Earth's umbral shadow is produced by ozone in Earth's stratosphere. In addition to being a strong ultraviolet absorber, ozone absorbs red light. The upper part of the moon is illuminated by refracted rays that have made a long slanting passage through our stratosphere. The reds towards the umbra centre are from light refracted through the denser troposphere. Image ©Eva seidenfaden, shown with permission. |
|
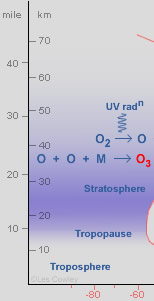
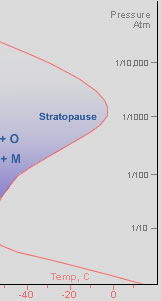
As we climb beyond the tropopause, temperatures start to increase again. We are now in the stratosphere, a region extending from a nominal 15 km (9 mile) up to the stratopause at 50 km (31 mile). Oxygen molecules, O2, iIn the upper stratosphere absorb short wavelength ultraviolet radiation (<200 nm) and dissociate into highly reactive oxygen atoms*. The atoms diffuse through the stratosphere and at heights of mostly 30-50 km many eventually combine** with more oxygen molecules to produce the reactive oxygen allotrope, ozone. Ozone, O3 ,is a strong absorber of longer wavelength (200-340 nm) UV radiation and the absorbed energy heats the atmosphere. The ozone layer is responsible for the stratosphere's increasing temperature with height***. Without ozone, mixing between the troposphere and stratosphere would be much faster and the structure of our atmosphere quite different. The ozone layer prevents harmful UV from reaching the earth’s surface and is partly responsible for the deep blue-violet beauty of the twilight sky.
|
|