OPOD
What's New
Rays & Shadows
Water Droplets
Rainbows
Ice Halos
High Atmosphere
Nacreous Clouds
Noctilucent Clouds
Rocket Trails
Airglow
About
More Images
Aurora
Zodiacal Light
Links & Resources
Search - Index
123456789012345678
| Airglow Formation |
|
|
|||||||||||||||
| The airglow is the light of electronically
and/or vibration-rotationally excited atoms and molecules 80 km or higher. |
|||||||||||||||
| Airglow vs Aurorae | Aurorae are at similar heights and are
also the light of excited atoms. There is a difference, however, auroral
excitation is by collisions with energetic particles whereas daytime short wavelength solar radiation produces the airglow via chemical excitation of which electronically excited oxygen atoms are the main component. |
||||||||||||||
| Production by sun's EUV radiation | The sun’s extreme
ultraviolet light excites oxygen and nitrogen atoms and molecules
in the thermosphere*.
The energetic products collide and interact with other atmospheric
components, including hydroxyl radicals (OH), to eventually produce
light emission by chemiluminescence** and
and decay of excited atoms and molecules. |
||||||||||||||
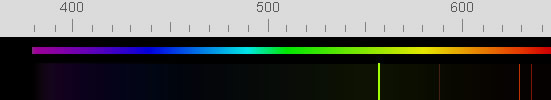
| Atomic oxygen green radiation |
The brightest emission is green 558nm
light from oxygen atoms in a layer 90-100 km high. The emission layer
is clearly visible from earth orbit. |
||||||||||||||
| radiation vs collisional de-excitation |
The excited atoms take
about a second to decay to another lower energy excited state***.
By atomic emission standards this is extremely slow and in that
time many excited atoms lose their energy instead by collisions,
mainly with nitrogen molecules. The emission does not occur at lower
altitude because the collisional quenching is so severe, the extreme
UV sunlight is less intense and there are fewer oxygen atoms |
||||||||||||||
| Atomic oxygen red light |
The red radiation of atomic
oxygen is from a lower energy excited state whose radiative half-life
is an immensely long, 110 seconds^.
This red airglow is only
found at 150 - 300 km where collisions are so infrequent that the
excited atoms have time to radiate away their energy. See also the
red emission from OH radicals below.. |
||||||||||||||
| Sodium | Another airglow component
is the familiar yellow light from sodium atoms^^ in a layer at 92
km.
|
||||||||||||||
| O2 | There are weak blue emissions
from excited molecular oxygen at ~95 km. |
||||||||||||||
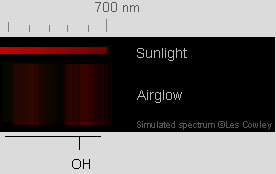
| OH | Vibrationally and rotationally excited OH radicals emit
red (image) and infra-red in a narrow layer (6-10 km FWHM) centered at ~ 86-87 km^^^. |
||||||||||||||
| Non uniformities | Airglow is not always
uniform. It can have bands and patches which shift and vary over
minutes. Gravity waves propagating from the lower atmosphere modulate
the atmospheric density, temperature and composition at airglow altitudes
and thus the airglow intensity. |
||||||||||||||
| Diurnal changes Solar 11 year cycle |
The airglow is brightest on Earth's day
side where the original excitation occurs. The night airglow is (fortunately!)
only one thousandth as bright and varies through the night. On a
much longer timescale the airglow varies with the 11 year cycle of
solar activity. |
||||||||||||||
|