We do not yet
know what shapes CO2 crystals actually have in Martian
clouds - but we can make reasonable predictions.
All crystals belong to one of only six symmetry systems. Water-ice in
Earth's cirrus clouds, Ice Ih, has hexagonal symmetry and
forms hexagonal prisms and pyramids. In contrast, solid carbon dioxide
has cubic symmetry.
This does not mean that CO2 crystallizes only into
microscopic cubes. Imagine walking around an enormously magnified crystal
where the individual atoms are visible. They lie in very orderly rows
and planes - in several particular directions there are clear avenues
and pathways through the lattice. These are the directions taken up by
the crystal faces. Facets running in other directions would entail an
overall increased energy and disorder and would be less stable. Thus each
of the six crystallogrphic symmetry systems in turn produces a limited
number of precise crystal forms. A cubic symmetry lattice
could give cubic, octahedral, twelve sided rhobic-dodecahedral and other
more complicated crystals. Combinations of these forms can
occur and so cuboctahedra, solids with six faces having the same direction
as a cube and eight more that of an octahedron, might also occur. As with
hexagonal water-ice, the crystals need not be regular - provided the interfacial
angles stay constant. The relative sizes of the faces can change to produce
a variety of habits of each form.
Microscopic CO2-ice was found as long ago as 1912
(ref 1) to have cubic, octahedral and cuboctahedral crystals and recently
(ref 2) they have been rephotographed with modern techniques. All these
could exist in clouds and rhombic dodecahedral crystals might also exist.
Each of these crystal forms might also have flat platelet type habits
- imagine the shape being made by machining down the opposite faces of
a regular crystal. As with Earth's crystals, the plate habits allow even
more fascinating halo possibilities!
Finally, CO2 is transparent at visible wavelengths
and is much more strongly refractive than water-ice. Armed with these
predictions and data it is quite possible to make accurate predictions
of possible halo in Martian skies. |
| |
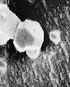
Recent electron micrographs of laboratory CO2 crystals.
At top is a cuboctahedron, the lower frame shows several octahedra. These
particular crystals are less than a micron across and would not form halos
and,of course, existence in the laboratory is no proof that these forms
occur in clouds! |
|
 |
| Ref.
1 |
(a)
H.E. Behnken, Phys. Rev. 35, 66-73 (1912)
(b) W. Wahl, Z. Physik. Chem. 88, 129-171 (1914) |
| Ref.
2 |
(a)
Wergin, W. P., J. L. Foster, A. T. C. Chang, D. K. Hall, A. Rango and
E. F.Erbe. 1997. Structure of carbon dioxide crystals (Martian snow) as
observed in TEM replicas and low temperature SEM images. Microsc. and
Microanalysis. (suppl 2): 1235-36.
(b) Foster, J. L., W. P. Wergin, E. Erbe, A. T. C. Chang, and D.
K. Hall. 1997.Observations and comparisons of H2O (snow) and CO2 crystals
using low-temperature scanning electron microscopy. Workshop on remote
sensing of planetary ices: Earth and other solid bodies. Flagstaff, AZ
June. |
