 |
|
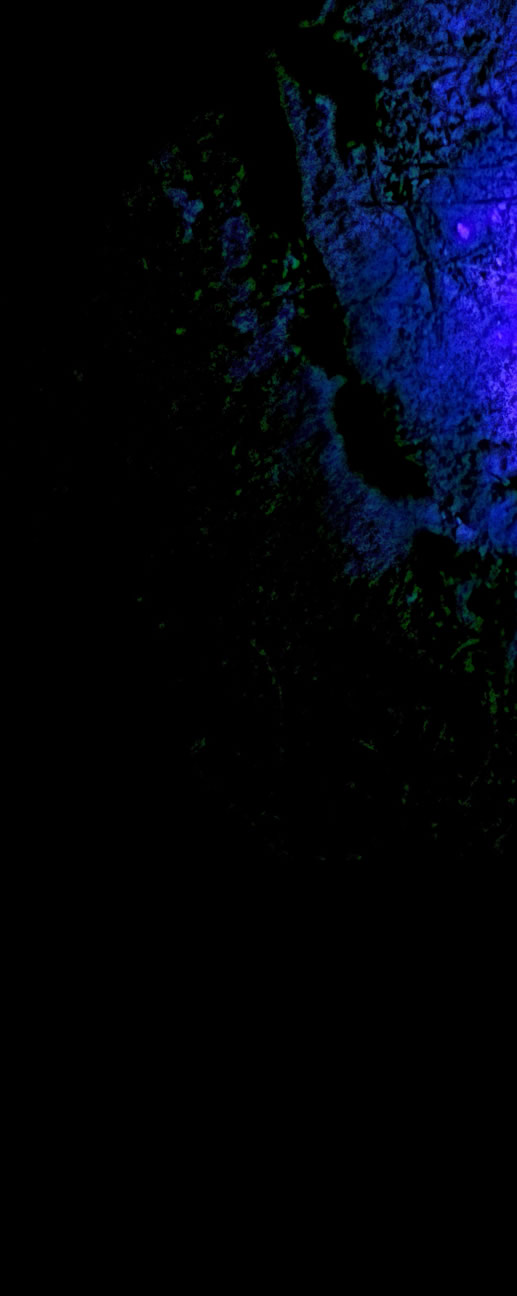
| Scorpion Fluorescence Imaged in the Negev Desert, Israel by Amir Bernat, Ilan Ramon youth physics center. Amir used a UV torch and 'phone camera in the dark to capture this yellow scorpion or deathstalker (leiurus quinquestriatus) fluorescing bright blue-green in the UV radiation. The yellow scorpion is the world's third most venomous and very dangerous - do not play with it! Image ©Amir Bernat, shown with permission. |
 |
|

| About - Submit | Optics Picture of the Day | Galleries | Previous | Next | Today |
 |
| Scorpions Scorpions like to shelter and hide. The carapaces of most if not all of them fluoresce blue-green when illuminated by ultra violet light. It is thought, but not known for certain, that they can sense the fluorescence so that they have in effect a whole body light collector to tell them when any part of them is unhidden and exposed to the night sky or moon. Scorpions with their many eyes masked still start to scurry about when lit by UV radiation (395nm). The UV is converted by fluorescence to cyan-green light which is then presumably detected by receptors in the carapace. |
| Fluorescence and more.. Go into an otherwise dark room illuminated by ultra-violet lamps. Teeth glow blue-white. Freshly laundered white shirts shine unnaturally blue-white with detergent whiteners. Some paper but not a banknote looks over-bright. Many minerals and gemstones have new colours. A molecule or crystal lattice is absorbing short wave radiation and becoming electronically excited. Two things can happen, the extra energy might be dissipated by collisions or other transfer routes (quenching) or it can be radiated away by an electronic transition to a lower energy state. The re-emitted radiation or fluorescence is usually � but not always � at longer (lower energy) wavelengths. Thus scorpion carapaces absorb short wavelength UV and fluoresce in longer wavelength blue-green. Phosphorescence is essentially fluorescence except that the light emission occurs over a longer timescale. The absorbed energy is transferred to electronic states which have very long radiative lifetimes because the necessary transitions for re-radiation are not allowed (forbidden in quantum mechanical parlance). The stored energy is eventually transferred to other states that are able to radiate and the delayed emission is phosphorescence. Chemiluminescence is quite different. A chemical reaction generates a new molecule in an excited state. The extra energy is then radiated away or is transferred to a another (dye) molecule that does so. Bioluminescence � the production of light by living things � is mostly a form of chemiluminescence from the oxidation of a molecule labelled a luciferin and catalysed by a specific enzyme (a luciferase). There are a whole variety of luciferin molecules that specifically power fireflies, squids, jellyfish, milky sea bacteria and more. |