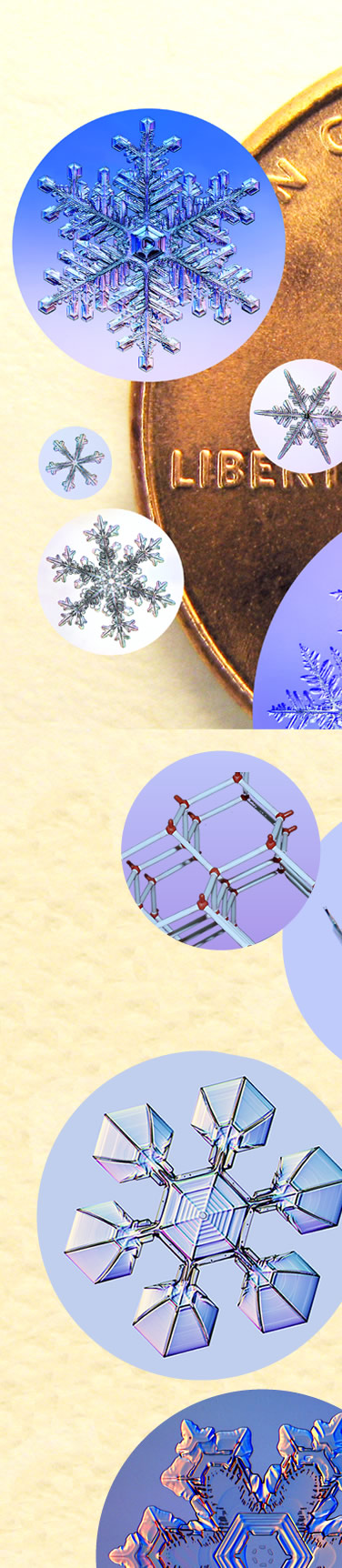
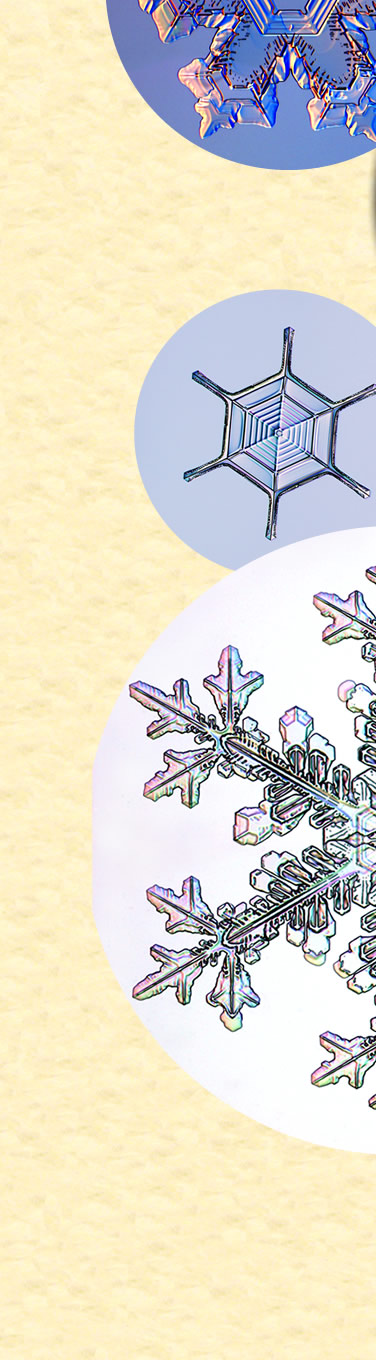
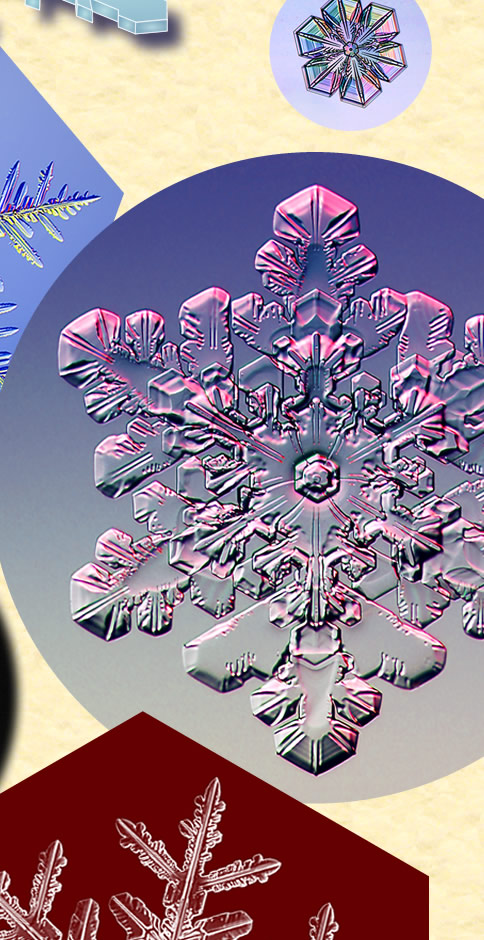
| Snow Crystals and a Penny
Kenneth Libbrecht, Professor of Physics at CalTech, collects snowflakes and grows his own. Here he compares them in size to a US Penny. “The largest crystal in that image is about 1 cm from tip to tip. I believe it still holds the record for the largest individual snow crystal ever photographed.” See his books “The Secret Life of a Snowflake”, “Field Guide to Snowflakes” and more. All images ©Kenneth Libbrecht, shown with permission |